Title: COMPUTATIONAL APPROACHES APPLIED TO DRUG DISCOVERY AND DOSAGE FORM DESIGNING.
Category: Faculty Development Program
Date: 8th& 12th Dec 2020 (5 days)
No. of Resource Persons: 14
No. of Participants: 100 as allowed
Organizers: Shri Ram Murti Smarak College of Engineering & Technology, Bareilly (Pharmacy)
Sponsored by: Dr. A. P. J. Abdul Kalam Technical University, Lucknow.
Mode: ONLINE
Objective:
This FDP on E-content development provides a platform to learn the tools and techniques needed for the design and development of digital resources for teaching and learning. It aims to provide the knowledge and skills needed for teachers to cater to the present day learners and their learning styles.
The Online Faculty Development Program was organized for five days 8th & 12th May 2020.
Day 1 started on 8th Dec 2020 at 9:30 am with Saraswati Vandana. Then Welcome Address was given by Prof. (Dr.) Prabhakar Gupta–Dean, SRMSCET, Bly who conveyed his best wishes for organizing such programme.


Then e-FDP Theme Presentation was briefed by Prof. (Dr.) Lalit Singh- Director SRMSCET Pharmacy who emphasized that the process of drug discovery is very complex as because out of 10,000 molecules only 1 molecule is established as a drug and requires an interdisciplinary effort to design effective and commercially feasible drugs. The conventional drug design methods include random screening of chemicals found in nature or synthesized in laboratories. The problems with this method are long design cycle that can vary from 1 year -20 years and high cost. Modern approach including structure-based drug design with the help of informatic technologies and computational methods has speeded up the drug discovery process in an efficient manner.
Then the Inaugural address was given by Chief Guest Prof. (Dr.) Devendra Pathak, Dean, Pharmacy College, U. P. University of Medical Sciences, Safai who said that the objective of drug design is to find a chemical compound that can fit to a specific cavity on a protein target both geometrically and chemically. After passing the animal tests and human clinical trials, this compound becomes a drug available to patients.
Then Address by Guest of Honor-Professor (Dr.) Jainendra Jain, Director, Ram-EESH Institute of Technical Education who said that He said that a systematic computational medicinal chemistry approach helps in research for treatment of various diseases.

Shri Aditya Murti Secretary, SRMS Trust said that Cutting edge chemical science technology and computer-aided drug design (CADD) is bringing together leading minds from across the world to help tackle the COVID-19 crisis. He also conveyed his best wishes to the organizing committee for conducting eFDP.

Finally the inaugural ceremony concluded with Vote of Thanks by Dr. Nita Yadav (e FDP Co ordinator).
Session 1 was delivered by Dr. Jainendra Jain Director, Ram-EESH Institute of Technical Education on topic “Structure based virtual screening & various Docking Molecules used in epilepsy Anti Epileptic Drug”. As an alternative to animal experiments, in vitro and in silico screening methods has been introduced to assist in the development of CNS active drugs. One of the major consequences of inadequate pharmacokinetics of both developmental and marketed drugs is failure in advanced development and/or market withdrawal. The balance between optimization of the physiochemical and pharmacokinetic properties to make the best in properties is critical for designing new drugs likely to penetrate the blood brain barrier and affect relevant biological systems. The modified analogues were docked with target protein shown good scores of bio affinity revealed that modified analogues can be further studied for synthesis and in vivo.
Session 2 was delivered by Dr. Manish Kumar Mishra, Director, Shri Guru Ram Rai University, Dehradun on topic “Computer Aided Drug Design”. He said that the process of drug development and drug discovery is very challenging, expensive and time consuming. It has been accelerated due to development of computational tools and methods. Over the last few years, computer aided drug design (CADD) also known as in silico screening has become a powerful technique because of its utility in various phases of drug discovery and development through various advanced features. In silico screening also paves path for the synthesis and screening of selected compounds for better therapeutics. computational chemistry and computer aided drug discovery which are aimed to cover a wide range of computational approaches including new methodologies as well as practical aspects in this area. This provides an insight about the developmental chain, approaches and applications of CADD; various data sources; computational methods for the discovery of new molecular entities; clinically approved drugs developed through CADD; and also summarizes the crucial steps of in silico drug designing like homology modelling, docking, multi-target searching and design, pharmacophore development, conformation generation and quantitative structure activity relationship (QSAR).
On Day 2 i.e. 9th Dec 2020 Session 1 was delivered by Dr. Ruchi Tiwari, Associate Professor from PSIT, Kanpur on topic “PBPK Simulation and Biowaiver study using IVIVC: Computational Approach using GastroPlusTM” .She said that the establishment of an in vitro–in vivo correlation (IVIVC) is considered the gold standard to establish in vivo relevance of a dissolution method and to utilize dissolution data in the context of regulatory bioequivalence questions, including the development of dissolution specifications. However, several recent publications, including industry surveys and reviews from regulatory agencies, have indicated a low success rate for IVIVCs, especially for immediate-release formulations. In recent years, the use of physiologically based pharmacokinetics (PBPK) and absorption modeling, as a tool to facilitate formulation development has been attracting increased attention. This manuscript provides an industry perspective on the current challenges with establishing IVIVCs and the potential PBPK and absorption modeling offer to increase their impact. Case studies across both immediate-release and extended-release formulations from five pharmaceutical companies are utilized to demonstrate how physiologically based IVIVC (PB-IVIVC) may facilitate drug product understanding and to inform bioequivalence assessment and clinically relevant specifications. Finally, PB-IVIVC best practices and a strategy for model development and application are proposed.
Session 2 was delivered by Dr. Gaurav Tiwari, Associate Professor from PSIT, Kanpur on topic “Artificial Neural Network & Artificial Intelligence in Drug Delivery and Pharmaceutical Research”. He said that artificial neural networks (ANNs) technology models the pattern recognition capabilities of the neural networks of the brain. Similarly to a single neuron in the brain, artificial neuron unit receives inputs from many external sources, processes them, and makes decisions. Interestingly, ANN simulates the biological nervous system and draws on analogues of adaptive biological neurons. ANNs do not require rigidly structured experimental designs and can map functions using historical or incomplete data, which makes them a powerful tool for simulation of various non-linear systems. ANNs have many applications in various fields, including engineering, psychology, medicinal chemistry and pharmaceutical research. Because of their capacity for making predictions, pattern recognition, and modeling, ANNs have been very useful in many aspects of pharmaceutical research including modeling of the brain neural network, analytical data analysis, drug modeling, protein structure and function, dosage optimization and manufacturing, pharmacokinetics and pharmacodynamics modeling, and in vitro in vivo correlations. He discussed the applications of ANNs in drug delivery and pharmacological research.
On Day 3 i.e. 10th Dec 2020 Session 1 was delivered by Dr. Kamla Pathak, Pharmacy College, Uttar Pradesh University of Medical School, Safai on the topic “Let’s talk about Pharmaceutical 3D printing”. She said that the pharmaceutical industry is moving ahead at a rapid pace. Modern technology has enabled the development of novel dosage forms for targeted therapy. However, the fabrication of novel dosage forms at industrial scale is limited and the industry still runs on conventional drug delivery systems, especially modified tablets. The introduction of 3D printing technology in the pharmaceutical industry has opened new horizons in the research and development of printed materials and devices. The main benefits of 3D printing technology lie in the production of small batches of medicines, each with tailored dosages, shapes, sizes, and release characteristics. The manufacture of medicines in this way may finally lead to the concept of personalized medicines becoming a reality. Her session provided an overview of how 3D printed technology has extended from initial unit operations to developed final product.
Session 2 was delivered by Dr. Jagdish Kumar Sahu, Assistant Professor, School of Pharmacy & Technology Management, SVKMS NMIMS, Shirpur, Maharashtra on the topic “Drug Repurposing: Novel approach for addressing COVID-19 outbreak”. He said that COVID-19 has now been declared pandemic and new treatments are urgently needed as we enter a phase beyond containment. Developing new drugs from scratch is a lengthy process, thus impractical to face the immediate global challenge. Drug repurposing is an emerging strategy where existing medicines, having already been tested safe in humans, are redeployed to combat difficult-to-treat diseases. While using such repurposed drugs individually may ultimately not yield a significant clinical benefit, carefully combined cocktails could be very effective, as was for HIV in the 1990s; the urgent question now being which combination.
Session 3 was delivered by Dr. Susheel Kumar, Professor & Director, School of Pharmaceutical Sciences, IFTM University, Moradabad on topic “Drug Like Properties: In drug Design & Discovery”. He said that the pharmaceutical industry is facing an ever increasing challenge to deliver safer and more effective medicines. Traditionally, drug discovery programs were driven solely by potency, regardless of the properties. As a result, the development of non-drug-like molecules was costly, had high risk and low success rate. To meet the challenges, the bar has been rising higher for drug candidates. They not only need to be active, but also drug-like to be advanced to clinical development. Drug-like properties, such as solubility, permeability, metabolic stability and transporter effects are of critical importance for the success of drug candidates. They affect oral bioavailability, metabolism, clearance, toxicity, as well as in vitro pharmacology. Insoluble and impermeable compounds can result in erroneous biological data and unreliable SAR in enzyme and cell-based assays. Rapid metabolism by enzymes and high efflux by transporters can lead to high clearance, short half-life, low systemic exposure and inadequate efficacy. Early property information helps teams make informed decisions and avoids wasting precious resources. Structure-property relationships are essential to guide structural modification to improve properties. High throughput ADME/TOX assays have been implemented and are being widely used to drive drug discovery projects in parallel with activity screening. Property design has become an integrated and inseparable part of the modern drug discovery paradigm. The approach has been proven to be a winning strategy.
On Day 4 i.e. 11th Dec 2020 Session 1 was delivered by Dr. Shardendu Kumar Mishra, Ram-eesh Institute of Vocational & Technical Education, Greater Noida on the topic “Drug Discovery & Development Process”. He said that Drug discovery is a process which aims at identifying a compound therapeutically useful in curing and treating disease. This process involves the identification of candidates, synthesis, characterization, validation, optimization, screening and assays for therapeutic efficacy. Once a compound has shown its significance in these investigations, it will initiate the process of drug development earlier to clinical trials. New drug development process must continue through several stages in order to make a medicine that is safe, effective, and has approved all regulatory requirements. One overall theme it is the process which is sufficiently long, complex, and expensive so that many biological targets must be considered for every new medicine ultimately approved for clinical use and new research tools may be needed to investigate each new target. From initial discovery to a marketable medicine is a long, challenging task. It takes about 12 – 15 years from discovery to the approved medicine and requires an investment of about US $1 billion. On an average, a million molecules screened but only a single is explored in late stage clinical trials and is finally made obtainable for patients. The whole session provided a brief outline of the processes of new drug discovery and development.
Session 2 was delivered by Dr. Vivek Kumar, Research Scientist, Integral Bioscientist Pvt. Ltd. on the topic “Identification of Potential Direct InhA Inhibitor for Isoniazid Resistant Tuberculosis: Insights from Computational studies”. He said that the InhA-related enoyl-ACP reductase, an enzyme involved in fatty acid synthesis, is one of the best validated targets for the development of anti-tubercular agents. However, the majority of isoniazid (INH)-resistant clinical strains are observed mainly due to the emergence of KatG mutants that do not form an INH-NAD adduct. Thus compounds that directly inhibit InhA avoiding activation by KatG would be promising candidates for combating MDR-TB. Herein, some predominant examples of InhA direct inhibitors recently developed were discussed and special attention was paid to 3D-structures of InhA in drug design process.

Session 3 was delivered by Dr. Sandeep Kumar Bansal, Associate Professor, Ram-eesh Institute of Vocational & Technical Education, Greater Noida on the topic “Computational Approaches applied to Drug Discovery and Dosage Form Designing”. He said that recent advances in combinatorial chemistry and high-throughput screening have made it possible for chemists to synthesize large numbers of compounds. However, this is still a small percentage of the total number that could be synthesized. Virtual screening encompasses a variety of computational techniques that allow chemists to reduce a huge virtual library to a more manageable size. His session provided the current state of the art in virtual screening and discusses approaches that will allow the evaluation of larger numbers of compounds.
On Day 5 i.e. 12th Dec 2020 Session 1 was delivered by Dr. Shiv Bharadwaj, Yeungnam University, Republic of Korea on topic “Introduction to Molecular Mechanics & Quantum Mechanics”. He said that Quantum chemical and molecular mechanics-generated structure and reactivity parameters comprise a part of chemo informatics, where such parameters are stored and properly indexed for search-information of a related molecule or a set of molecular systems. These could be used for quantitative structure activity relation (QSAR) modeling. He discussed the applicability of various quantum chemical techniques for such property (QSAR parameters) and density functional theory (DFT)-related techniques have been advocated to be quite useful for such purposes. Molecular mechanics methods, although mostly useful for less time consuming structure calculations and important in higher level molecular dynamics and Monte-Carlo simulations, are sometimes useful to generate structure-related descriptors for QSAR analysis. His discussion in this connection with molecular mechanics-related QSAR modeling is included to show the use of such descriptors was very informative.
Session 2 was delivered by Dr. Rabi Sankar Bhatta, CSIR-CDRI, Lucknow on the topic “Computer Aided Biopharmaceutical Characterization”. He said that parameters used for model construction and the sensitivity predicted pharmacokinetic responses to various input parameters are described. Virtual trials for in silico modeling of drug absorption are presented. The influences of food on drug absorption, as well as correlation between the in vitro and in vivo results, are also addressed, followed by biowaiver considerations.
Session 3 was delivered by Dr. Vikas Mishra, Assistant Professor, Depart of Pharmaceutical Sciences, BBAU, Lucknow on the topic “Open Source Software’s and Web Services for Drug Discovery Research”. He said that despite the tremendous progress in the field of drug designing, discovering a new drug molecule is still a challenging task. Drug discovery and development is a costly, time consuming and complex process that requires millions of dollar and 10-15 years to bring new drug molecules in the market. This huge investment and long-term process are attributed to high failure rate, complexity of the problem and strict regulatory rules, in addition to other factors. Given the availability of ‘big’ data with ever improving computing power, it is now possible to model systems which is expected to provide time and cost effectiveness to drug discovery process. Computer Aided Drug Designing (CADD) has emerged as a fast alternative method to bring down the cost involved in discovering a new drug. In past, numerous computer programs have been developed across the globe to assist the researchers working in the field of drug discovery. Broadly, these programs can be classified in three categories, freeware, shareware and commercial software. In this review, we have described freeware or open-source software that is commonly used for designing therapeutic molecules. Major emphasis will be on software and web services in the field of chemo- or pharmaco-informatics that includes in silico tools used for computing molecular descriptors, inhibitors designing against drug targets, building QSAR models, and ADMET properties.
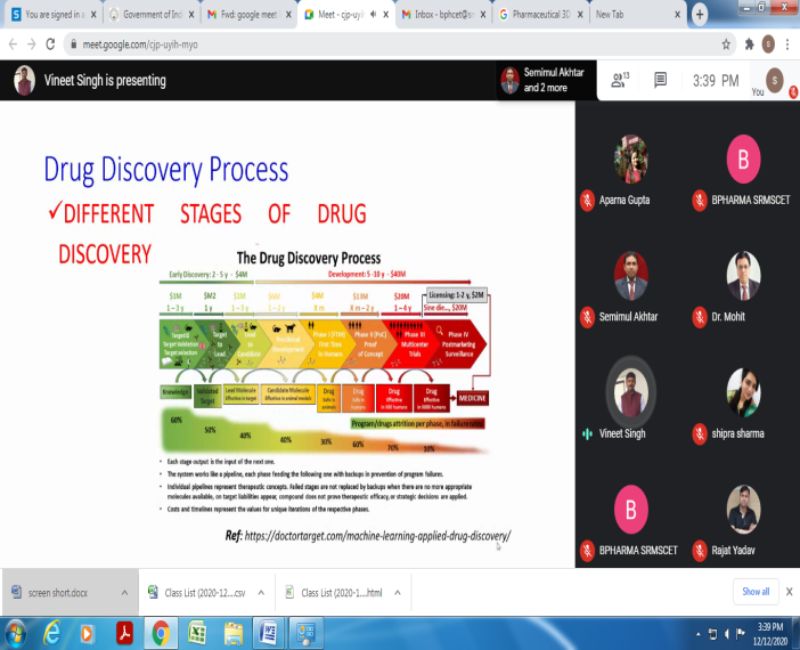
Session 4 was delivered by Dr. Vineet Singh, Shri Govind Ram SGSITS, Indore on the topic “Drug Design & Development”. He said that Drug design, also known as rational drug design, is the inventive process of finding new medications based on the knowledge of a biological target. Drug design defines the design of molecules that are complementary in shape and charge to the bimolecular target with which they interact and therefore will bind to it. Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. His session covered the following points: Ligand based drug designs; Structure based drug designs, Pre-clinical phase, Clinical phase, Valuation, Novel initiatives& Success rate of the same.
Meanwhile, the valedictory session witnessed participation of over 100 delegates and invited dignitaries. Dr Mohit Gupta delivered the welcome address and presented a brief summary of takeaways in terms of lessons and recommendations. The participants also shared their experience and learning of the whole programme.



